How Could You Separate Magnesium Chloride From Silver Chloride
Silver chloride is a powder that sits at the bottom of the Mgcl2 solution. Start with a balanced equation.

How Could You Separate Magnesium Chloride From Silver Chloride Lisbdnet Com
The two can be separated through filtration.

. Weigh your mixture of TeO2 and SiO2. Kimdelivenge1303 kimdelivenge1303 02122018 Chemistry Secondary School answered How could you separate magnesium chloride from silver chloride. 6H 2 ONaCl to be referred to as MS using a dense media of such organic solvents as tetrachloromethane iodomethane and a combination of these two solvents.
Silver chloride is a powder that sits at the bottom of the Mgcl2 solution. Upon adding SO4 2- to BaCl2. Silicon dioxide on the other hand is soluble in Hydroflouric acid.
This is the best answer based on feedback and ratings. Get the answers you need now. The unique insolubility of silver chloride makes it possible to readily achieve a distinctive separation of silver from a mixed metal ion acidic solution.
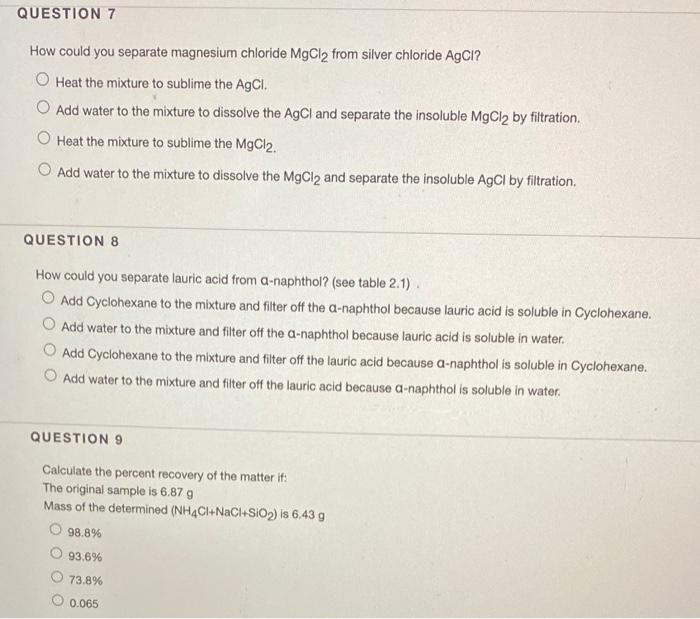
QUESTION 7 How could you separate magnesium chloride MgCl2 from silver chloride AgCl. How could you separate magnesium chloride MgCl2 from silver chloride AgCl. This is the easiest and most widely used way of separation of magnesium.
5 How could you separate lauric acid from. 1 See answer kimdelivenge1303 is waiting for your help. Add water to the mixture to dissolve the AgCl and separate the insoluble MgCl2 by filtration Heat the mixture to sublime the MgCl2.
Using chloride precipitation with NaCl solution the plant operation selectively removes all the silver from the pressure leach solution. Previous question Next question. Silver chloride AgCl can be separated from magnesium chloride MgCl2 by the method of precipitation.
Decant your acid solution. Solid barium sulfate is a solid white precipitate. Learn this topic by watching Solubility Rules Concept Videos.
Dry and weigh the remaining Te02. How could you separate magnesium chloride MgCl2 from silver chloride AgCl. 100 2 ratings AgCl is insoluble in water.
To separate out a mixture containing ammonium chloride sodium chloride and magnesium carbonate we can use their properties specifically water solubility and decomposition behavior. How could you separate tellurium oxide TeO2 from SiO2. This is a limiting reagent question.
How could you separate magnesium chloride MgCl 2 from silver chloride AgCl. All Organic Chemistry Practice Problems Solubility Rules Practice Problems. How could you separate magnesium chloride from silver chloride.
Add your answer and earn points. The solid MS mixture to be separated was prepared from two different sources. Submerge in hydroflouric acid.
See the answer See the answer done loading. Silver Chloride Precipitation Separation. MgCl2 is aqueous.
The major difference between magnesium chloride MgCl2 and silver chloride AgCl is. Experimental results are reported for the solid-solid separation of a mixture of MgCl 2. Tellerium dioxide is not soluble in water or acid.
Allow the SiO2 to disolve. 2Li CaCl₂. How could you separate magnesium chloride from silver chloride.
O Heat the mixture to sublime the AgCl. How could you separate magnesium chloride from silver chloride. So all you have to do it pour the liquid off into a separate beaker and let the powder dry.
How can you separate magnesium chloride and silver chloride. How could you separate magnesium chloride from silver chloride. How could you distinguish solid barium chloride from solid barium sulfate.
So all you have to do it pour the liquid off into a separate. Silver chloride is a powder that sits at the bottom of the Mgcl2 solution. Silver chlorideAgCl can be separated from magnesium chlorideMgCl2 by the method of precipitation.
How Could You Separate Magnesium Chloride From Silver ChlorideSilver chlorideAgCl can be separated from magnesium chlorideMgCl2 by the method of precipitation. See all problems in Solubility Rules. How could you separate magnesium chloride MgCl2 from silver chloride AgC 4.
Silver chloride is a powder thatsits at the bottom of the Mgcl2 solution. How could you separate magnesium chloride from silver chloride. Since MgCl2 is highly soluble in water due to its polar nature and AgCl is negligibly soluble in water due to its dominating non-polar nature they can be separated by making an aqueous.
Magnesium chloride is soluble in water and can be separated by filtration from AgCl. How Could You Separate Magnesium Chloride From Silver Chloride. More details Get Price.
So all you have to do itpour the liquid off into a separate beaker and let the powderdry. Since MgCl2 is highly soluble in water due to its polar nature and AgCl is negligibly soluble in water due to its dominating non-po. So all you have to do it pour the liquid off into a separate beaker and let the powder.
Silver chloride sits at the bottom so you can just filter it out. How could you separate barium sulfate BaSO4 from NaCI. How could you separate tellurium dioxide TeO2 from Sio2.
AgCl is insoluble while MgCl2 is soluble so they can be seperated by filtration and recrystallisation can be used to get solids. Since MgCl2 is highly soluble in water due to its polar nature and AgCl is negligibly soluble in water due to its dominating non-polar nature they can be separated by making an aqueous solution. The reactant that produces the least number of moles of a product is the limiting reagent and determines the maximum amount of a product that can be produced in the reaction.
So all you have to do it pour the liquid off into a separate. MgCl2 aq 2 AgNO3 aq ----- MgNO32 2 AgCl s There is a white. View the full answer.
Solved Question 7 How Could You Separate Magnesium Chloride Chegg Com

How Could You Separate Magnesium Chloride From Silver Chloride Lisbdnet Com

How Could You Separate Magnesium Chloride From Silver Chloride Lisbdnet Com
0 Response to "How Could You Separate Magnesium Chloride From Silver Chloride"
Post a Comment